Ethical Issues in Artificial intelligence (AI) Based Clinical Trials
Clinical trials in the modern period raise a number of moral and ethical concerns. We need to look at AI-based clinical trials from a range of different moral vantage points if we want to increase their effectiveness in terms of human rights, human value issues, and human safety.
“Doing no harm and uplifting human freedom, values, and rights are the core aspects of ethical AI systems – Sri Amit Ray

The testing of novel medications in humans can give rise to ethical questions, such as those pertaining to efficacy, safety, and informed appropriate consent. There are a number of additional ethical concerns that may surface during clinical studies, including the following:
- Informed consent: Patients must give informed consent for their participation in a clinical trial, meaning that they understand the potential risks and benefits of the treatment being tested. However, there may be cases where patients are not fully informed or do not fully understand the risks and benefits, which raises ethical concerns.
- Approval of the consent: To stop exploitation, the consent should be approved by the family members and the well wishers of the patients and the third-party of the society.
- Placebo-controlled trials: Some clinical trials use a placebo control group, which means that some patients will not receive the active treatment being tested. This raises ethical concerns, as patients in the control group may not receive the best available treatment for their condition.
- Exploitation of vulnerable populations: Clinical trials are often conducted in developing countries or in populations that are considered vulnerable, such as prisoners or people with mental illness. There is a risk that these populations may be exploited or not fully informed about the risks and benefits of the trial.
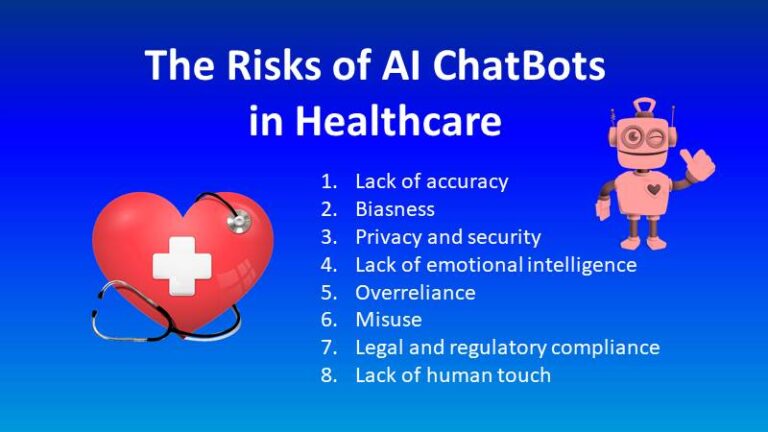
- Data privacy and security: Clinical trials generate a large amount of sensitive patient data, which raises concerns about data privacy and security.
- Safety and efficacy: Clinical trials are intended to determine the safety and efficacy of a treatment, however, there may be cases where the risks outweigh the benefits, or the trial result is not conclusive. In such cases, there are ethical concerns about the continuation of the trial.
Ethical issues in Drug Discovery
There are several ethical issues that can arise in drug discovery, including:
- Animal testing: Many drug candidates are tested on animals before they are tested on humans. This raises ethical concerns about the use of animals in research and the potential suffering of the animals.
- Human clinical trials: Clinical trials of new drugs can raise ethical concerns, such as informed consent, safety, and efficacy, as I previously mentioned.
- Access to medicines: There is a concern that the high cost of developing new drugs may lead to limited access to those medicines for patients who need them, particularly in low- and middle-income countries.
- Intellectual property rights: Pharmaceutical companies often hold patents on new drugs, which can lead to high prices and limited access to the medicines.
- Conflict of interest: Pharmaceutical companies may have a financial interest in the outcome of drug discovery research, which can raise ethical concerns about the integrity of the research.
- Off-label use: Some drugs may be used in ways other than they are officially approved for, which raises ethical concerns about the safety and efficacy of these uses.
Conclusion:
In general, it is essential that the process of drug discovery be carried out in a manner that is both ethical and responsible, with the health and safety of patients serving as the primary focus. This includes making sure that the use of animals in experiments is kept to a minimal, that clinical trials are carried out in a safe and ethical manner, that access to medicines is fair, and that conflicts of interest are detected and correctly addressed.
It is important for clinical trials to be conducted in an ethical and responsible manner, with the well-being of the patient as the primary concern. This includes ensuring that patients are fully informed about the risks and benefits of the trial, that the risks of the trial do not outweigh the benefits, and that patient data is handled in a way that respects privacy and security.